Welcome to Aptra Synthesis Pvt Ltd
Welcome to Aptra Synthesis Private Limited, a reliable and vertically integrated manufacturer of APIs and intermediates, boasting state-of-the-art manufacturing facilities that adhere to GMP standards. We take great pride in producing top-notch intermediates through our fully backward-integrated process.
Aptra Synthesis Private Limited (ASPL) stands as one of the rapidly emerging manufacturers of Active Pharmaceutical Ingredients (APIs), Pellets (Semi-finished Formulations), and Intermediates. Our establishment was founded with a core vision: to become the foremost preferred API supplier in the global pharmaceutical market. Our relentless pursuit of this vision hinges on a dual commitment to quality and affordability.
At Aptra Synthesis Private Limited, we drive this commitment by drawing upon deep-seated industry expertise, a highly qualified team of professionals characterized by their integrity, and advanced infrastructure. These factors, in turn, enable us to forge enduring and robust business partnerships on a global scale.
Aptra Synthesis Private Limited (ASPL) is strategically poised to accommodate substantial production capacity, meticulously aligned with international and national regulatory standards. Moreover, our production capacity holds the potential for substantial scalability, ensuring adaptability to future demands.
Our overarching vision and mission revolve around furnishing premium-quality products that deliver unparalleled value, all while upholding superior customer service that remains accessible around the clock.
Aptra Synthesis Private Limited (ASPL) is one of the fastest growing manufacturers of Active Pharmaceutical Ingredients (APIs), Pellets (Semi finished Formulations) and Intermediates. Aptra Synthesis Private Limited was established with a main vision of achieving “the most preferred API supply partner to the pharmaceutical market worldwide”. At Aptra Synthesis Private limited we aim to achieve this vision with a clear focus on quality & affordability. Our strong commitment of providing high quality products is boasted by in-depth industry knowledge, well- qualified team of professionals with integrity, as well as advanced infrastructure and as a result, creating strong and long-lasting business partnerships worldwide Aptra Synthesis Private Limited (ASPL) is planned to accommodate huge production capacity aligning with International and National regulatory norms. There is scope to scale up the production capacity to huge quantities in future, if necessary. Our vision and mission is to provide high quality products at a best-in-class value with superior customer service around the clock.
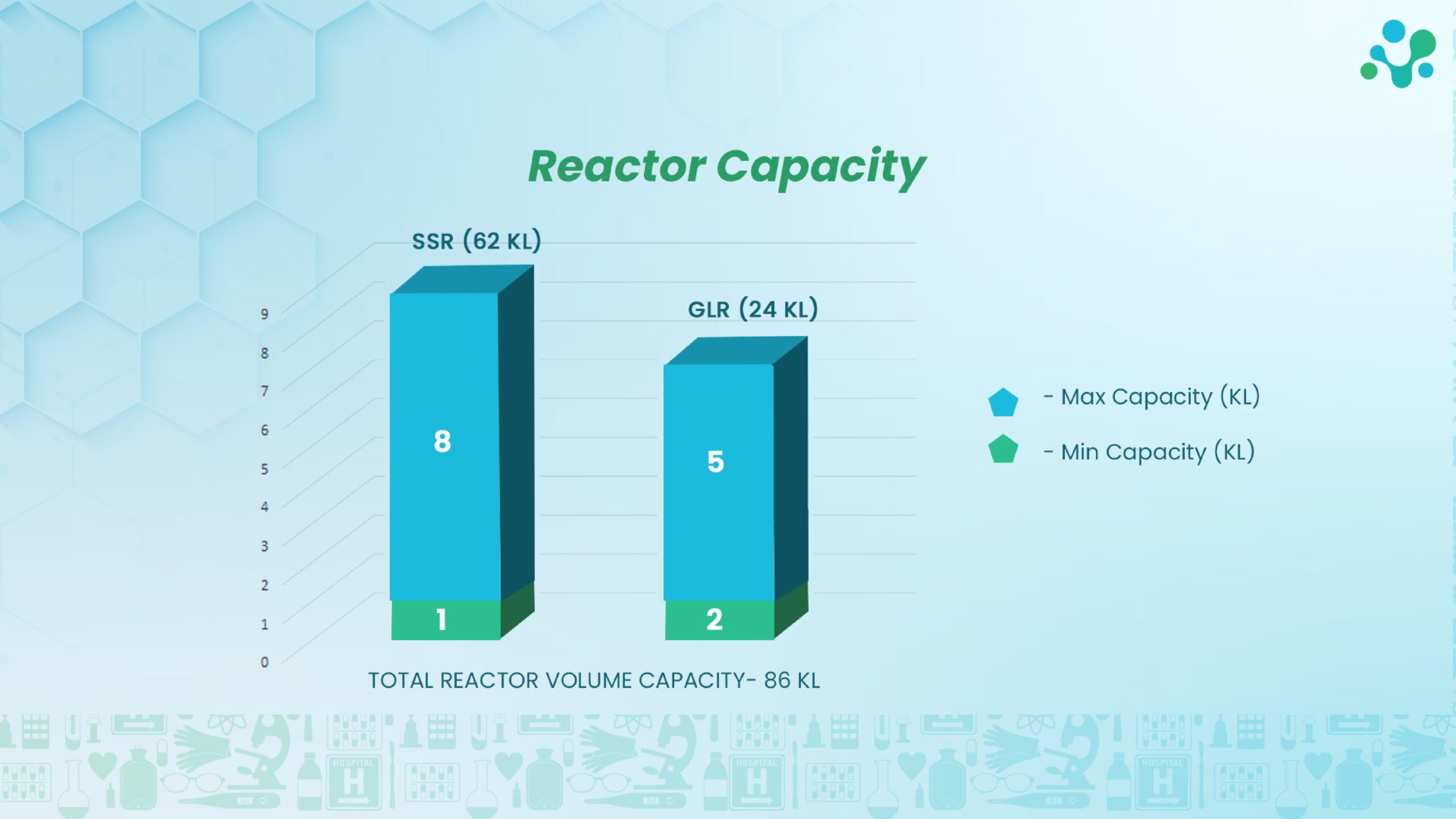
Production Equipment
- - 15 SSR
- - 8 GLR
- - 4 Centrifuge
- - 5 TRAY DRYERS
- - 3 RCVD
- - 3 VIBRO SIFTER
QA/QC INSTRUMENTS
HPLC
TOTAL NO. - 3
Agilent with EZ chrome software – 02 no.
waters with empowers 2 software – 01 no.
GC
TOTAL NO. - 2
GC auto injector (Agilent) with EZ chrome elite – 01 no.
GC-HS (Simadzu) with lab solution software – 01 no.
Stability Chamber
TOTAL NO. - 2 Make - Therolab
Long term storage condition: 25 + 2°c & 60% + 5% Rh
Accelerated storage condition: 40 + 2°c & 75% + 5% Rh
Other Instruments
FT – IR spectrophotometer – 1 no.
UV visible spectrophotometer – 1 no.
Utilities
| Boiler | 2.0 Ton/hr (135°c) |
| Generator | 225 KVA (100% Power Backup) |
| Chilling Plant | 60TR (02 no.) at -20°C |
| Purified water System | 1 KL/hr |
| Vacuum System | High/generaI vacuum |
| Hot water System | 20 m2 /hr |
| Cooling Tower | 300 TR, 200 TR, 200 TR |
| Air Compressor | 115 CFM |
Reaction CapabiIities
REACTION CARRIED OUT AT SITE:
– CHLORINATION
– BROMINATION
– HYDROGINATION
– EPOXIDATION
– HYDROLYSIS
– REDUCTION
– CONDENSATION
– METHYLATION
– FRIEDEL CRAFT REACTION
TEMP. MAX.: +250°C
TEMP. MIN.: -40°C
Clean Room
– Site having 3 air handling unit i.e. AHU-101, AHU-J02 and AHU-103 respectively.
– AHU-JO1 is for Pharma crystolliser area.
– AHU-JO2 is for Pharma powder processing area.
– AHU-JO3 is for Pharma pilot plant.
– AHU pre-filters ore 10 p, 5 p and terminally HEPA filter.
– AHU system validated.
– There is no air re-circulation.
– Area classified as class 1,00,000
Quality Management System
- Quality Management System is as per ICH guideline.
- All changes ore routed through change control procedure.
- Deviation procedure is in place.
- Annual product quality review is in place.
- Batch release procedure is in place.
- Vendor qualification procedure is in place.
- Market complaint procedure is in place.
- Corrective and Preventive Action procedure is in place.
Our Manufacturers








Infrastructure
Production Capabilities
As a leading API manufacturer and Contract Development and Manufacturing Organization (CDMO) partnering both domestically in India and internationally, we specialize in API manufacturing through complete backward integration. Our extensive suite of services includes API development and Commercial Manufacturing for Innovators and Formulators. We effortlessly scale from grams to multi-tonnage, adhering strictly to cGMP norms.
Reach us
India Office address:
Plot no 36/B, Aptra Towers, TSIIC Industrial Park, Balanagar, Hyderabad.